Introduction to Oral Semaglutide
Oral semaglutide is a groundbreaking medication in the management of type 2 diabetes, representing a significant advancement in diabetes treatment. This medication is part of a class of drugs known as GLP-1 receptor agonists, which are traditionally administered via subcutaneous injection. Oral semaglutide, however, is the first in this class to be available in a pill form, offering a more convenient option for patients. This article will explore the development, mechanism of action, clinical efficacy, safety profile, and the impact of oral semaglutide on the management of type 2 diabetes.
Development of Oral Semaglutide
The development of oral semaglutide is a remarkable achievement in pharmaceutical innovation. Traditionally, GLP-1 receptor agonists have been administered through injections because GLP-1 (glucagon-like peptide-1) is a peptide hormone that is rapidly degraded in the gastrointestinal tract. The breakthrough came with the use of an absorption enhancer called SNAC (sodium N-(8-[2-hydroxybenzoyl]amino) caprylate). SNAC facilitates the absorption of semaglutide through the stomach lining, allowing it to enter the bloodstream effectively.
Novo Nordisk, the company behind semaglutide, conducted extensive research and numerous clinical trials to bring this oral formulation to market. The goal was to provide an effective and convenient treatment option that could improve adherence and outcomes for patients with type 2 diabetes. The culmination of this research was the approval of oral semaglutide by the U.S. Food and Drug Administration (FDA) in September 2019 under the brand name Rybelsus.
Mechanism of Action
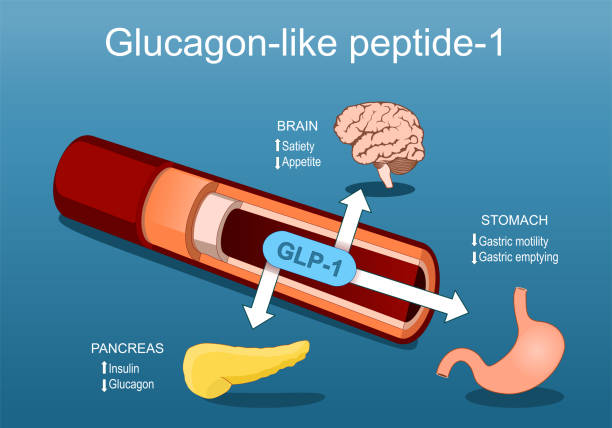
Oral semaglutide works by mimicking the effects of GLP-1, a hormone that plays a crucial role in glucose metabolism. GLP-1 receptor agonists like semaglutide bind to GLP-1 receptors on pancreatic beta cells, stimulating insulin secretion in response to meals. Additionally, these drugs suppress glucagon release, slow gastric emptying, and promote satiety, all of which contribute to better glycemic control.
Enhanced Insulin Secretion
One of the primary actions of semaglutide is to enhance insulin secretion. When blood glucose levels rise after a meal, semaglutide stimulates the pancreatic beta cells to release insulin, helping to lower blood glucose levels. This is particularly beneficial for individuals with type 2 diabetes, who often have impaired insulin secretion.
Inhibition of Glucagon Release
Semaglutide also inhibits the release of glucagon, a hormone that raises blood glucose levels by promoting the release of glucose from the liver. By suppressing glucagon, semaglutide helps to prevent excessive glucose production by the liver, thereby contributing to better glycemic control.
Slowed Gastric Emptying
Another important effect of semaglutide is the slowing of gastric emptying. This means that food moves more slowly from the stomach to the small intestine, leading to a more gradual absorption of glucose into the bloodstream. This effect helps to prevent sharp spikes in blood glucose levels after meals.
Promotion of Satiety
Semaglutide promotes a feeling of fullness, or satiety, which can help individuals with type 2 diabetes to eat less and potentially lose weight. This is particularly important because obesity is a major risk factor for the development and progression of type 2 diabetes.
Clinical Efficacy
The clinical efficacy of oral semaglutide has been demonstrated in several large-scale clinical trials. These trials have shown that oral semaglutide is effective in improving glycemic control and promoting weight loss in patients with type 2 diabetes.
PIONEER Clinical Trials
The PIONEER (Peptide Innovation for Early Diabetes Treatment) program was a series of clinical trials designed to evaluate the safety and efficacy of oral semaglutide. The program included 10 trials, each with a specific focus, such as comparing oral semaglutide to placebo, other GLP-1 receptor agonists, or standard treatments like metformin and insulin.
PIONEER 1
In the PIONEER 1 trial, oral semaglutide was compared to a placebo in patients with type 2 diabetes who were not adequately controlled with diet and exercise alone. The results showed that oral semaglutide significantly reduced HbA1c levels (a measure of long-term blood glucose control) and body weight compared to placebo.
PIONEER 2
The PIONEER 2 trial compared oral semaglutide to empagliflozin, an SGLT-2 inhibitor, in patients with type 2 diabetes. Oral semaglutide demonstrated superior reductions in HbA1c and body weight compared to empagliflozin.
PIONEER 3
In the PIONEER 3 trial, oral semaglutide was compared to sitagliptin, a DPP-4 inhibitor, in patients with type 2 diabetes. The results showed that oral semaglutide was more effective in reducing HbA1c levels and promoting weight loss than sitagliptin.
PIONEER 4
The PIONEER 4 trial evaluated the efficacy of oral semaglutide compared to liraglutide (a subcutaneous GLP-1 receptor agonist) and placebo. Oral semaglutide was found to be non-inferior to liraglutide in terms of HbA1c reduction and was superior to placebo.
PIONEER 5
In the PIONEER 5 trial, the safety and efficacy of oral semaglutide were assessed in patients with type 2 diabetes and moderate renal impairment. The results indicated that oral semaglutide was effective and well-tolerated in this patient population.
PIONEER 6
The PIONEER 6 trial was a cardiovascular outcomes trial designed to assess the cardiovascular safety of oral semaglutide. The trial showed that oral semaglutide did not increase the risk of major adverse cardiovascular events compared to placebo, thereby confirming its cardiovascular safety.
Safety Profile
The safety profile of oral semaglutide has been well-studied in clinical trials. The most common adverse effects are gastrointestinal in nature and include nausea, vomiting, and diarrhea. These side effects are generally mild to moderate in severity and tend to decrease over time as the body adjusts to the medication.
Gastrointestinal Effects
Nausea is the most frequently reported side effect of oral semaglutide. It usually occurs early in the treatment and tends to diminish over time. To minimize the risk of nausea, it is recommended to start with a lower dose and gradually increase it. Vomiting and diarrhea are also common but usually resolve with continued use of the medication.
Risk of Hypoglycemia
Oral semaglutide has a low risk of causing hypoglycemia (low blood sugar) when used as monotherapy. However, the risk of hypoglycemia increases when oral semaglutide is used in combination with other medications that lower blood glucose levels, such as insulin or sulfonylureas. Patients should be educated about the symptoms of hypoglycemia and how to manage it.
Cardiovascular Safety
The cardiovascular safety of oral semaglutide was confirmed in the PIONEER 6 trial, which showed that the medication did not increase the risk of major adverse cardiovascular events. This is an important consideration for patients with type 2 diabetes, who are at an increased risk of cardiovascular disease.
Other Considerations
Other potential side effects of oral semaglutide include decreased appetite, indigestion, and constipation. In rare cases, patients may experience more serious side effects, such as pancreatitis or kidney problems. It is important for patients to discuss any concerns or side effects with their healthcare provider.
Impact on Diabetes Management
Oral semaglutide has the potential to significantly impact the management of type 2 diabetes by providing a convenient and effective treatment option. The availability of an oral GLP-1 receptor agonist may improve patient adherence to medication, leading to better glycemic control and improved outcomes.
Convenience and Adherence
One of the main advantages of oral semaglutide is its convenience. Many patients prefer oral medications over injections, and the availability of an oral GLP-1 receptor agonist can improve adherence to treatment. Better adherence to medication is associated with improved glycemic control and a reduced risk of diabetes-related complications.
Weight Management
Weight management is a critical component of diabetes care, and oral semaglutide has been shown to promote weight loss in clinical trials. This is particularly important for patients with type 2 diabetes, as obesity is a major risk factor for the development and progression of the disease. By promoting weight loss, oral semaglutide can help to improve overall health and reduce the risk of complications.
Cardiovascular Benefits
In addition to improving glycemic control, GLP-1 receptor agonists like semaglutide have been shown to have cardiovascular benefits. The cardiovascular safety of oral semaglutide has been confirmed, and there is evidence to suggest that it may help to reduce the risk of cardiovascular events in patients with type 2 diabetes. This is an important consideration, as cardiovascular disease is a leading cause of morbidity and mortality in this patient population.
Patient and Provider Perspectives
The introduction of oral semaglutide has been met with enthusiasm from both patients and healthcare providers. For patients, the availability of an effective oral medication offers a more convenient option and can improve adherence to treatment. Healthcare providers appreciate the ability to offer a new treatment option that can help to achieve better glycemic control and promote weight loss.
Patient Experience
Patients who have switched to oral semaglutide often report a positive experience. Many find it easier to take a pill once daily compared to managing injections, and the improved convenience can lead to better adherence to treatment. Additionally, the weight loss benefits of oral semaglutide are often.